Container Closure System Integrity Testing

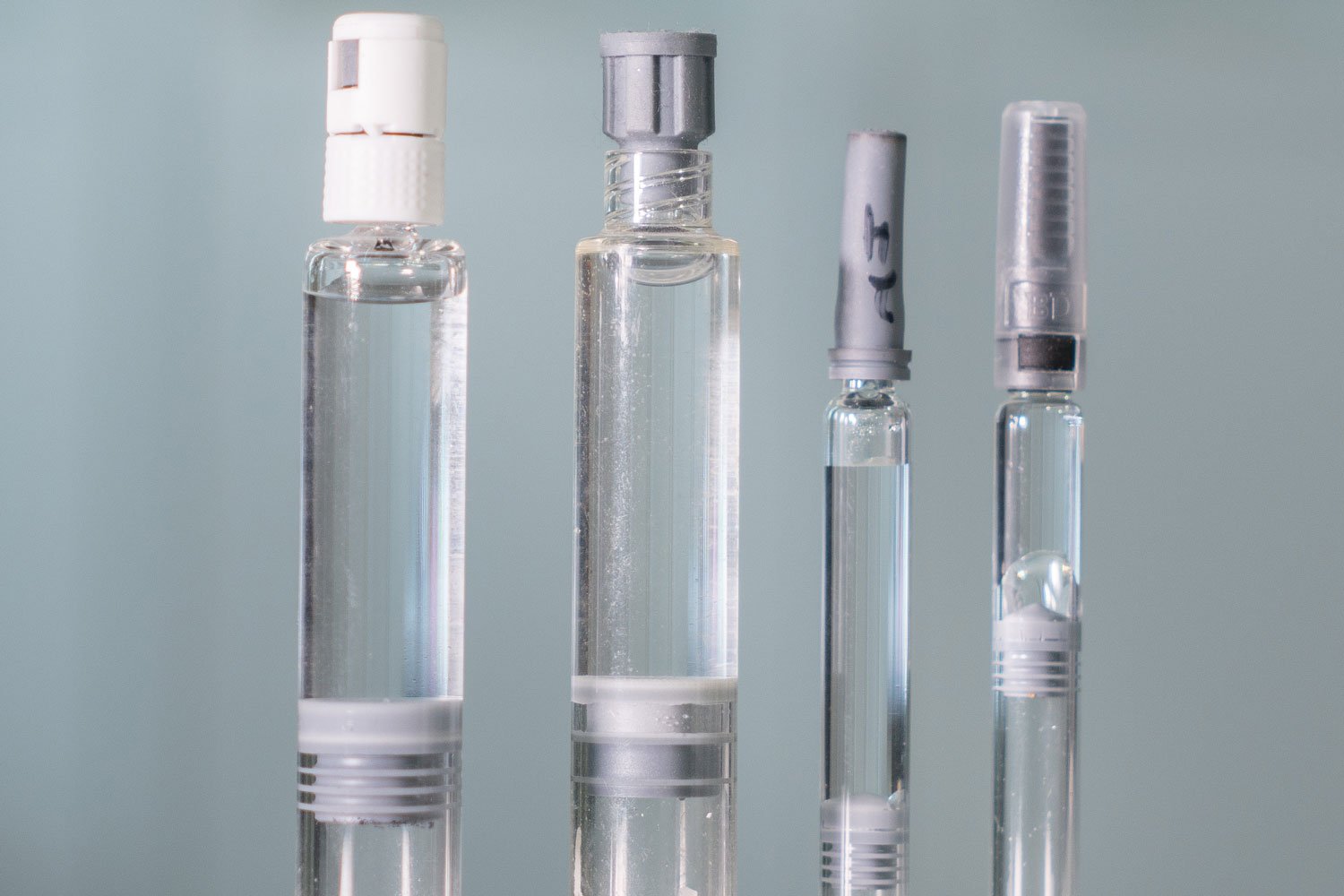
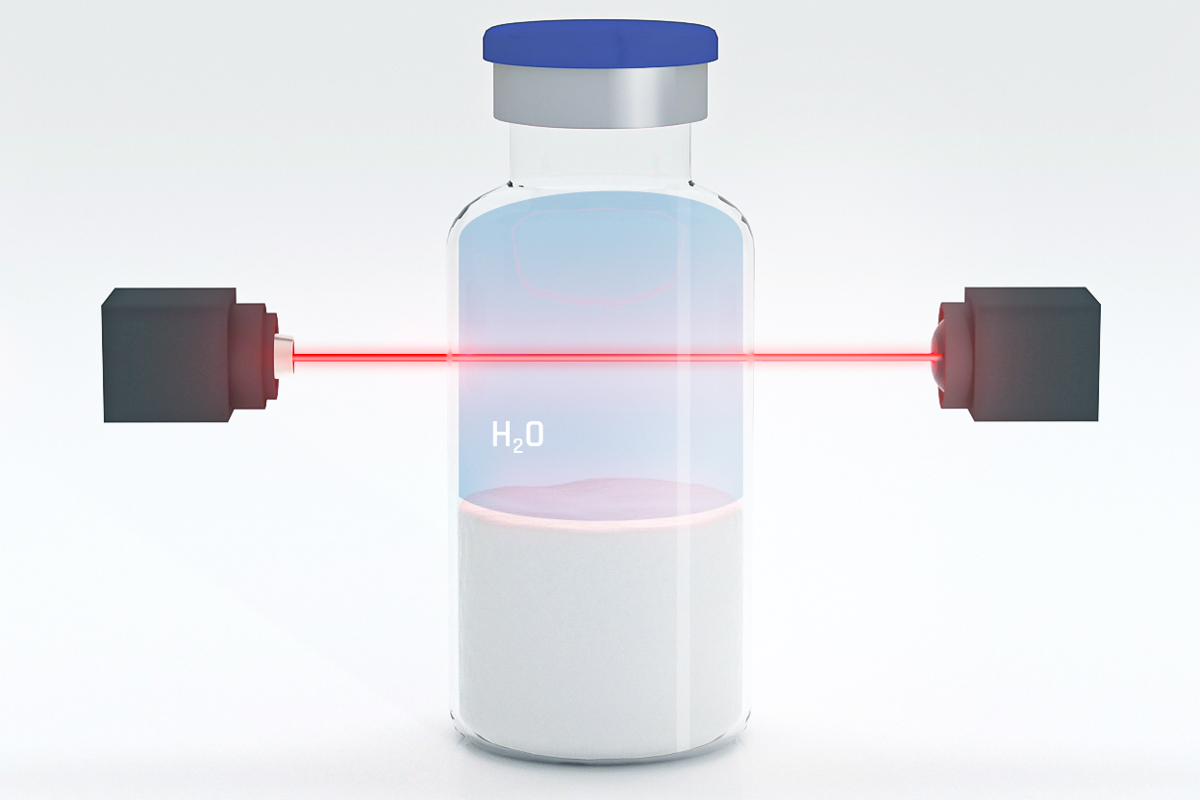
Container closure integrity testing ccit by dye ingress and microbial challenge rigid containers are often tested as part of a stability study to ensure that the closure of the container can maintain a sterile barrier.
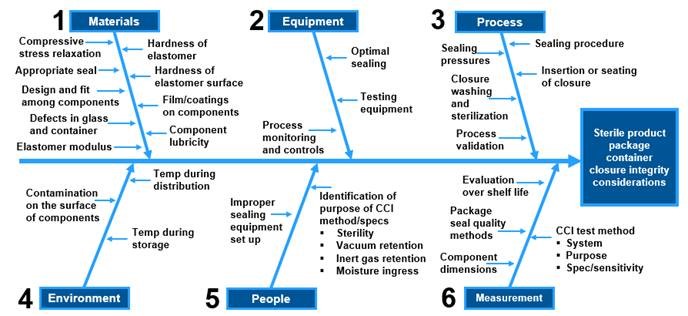
Container closure system integrity testing. Practical aspects and approaches in the pharmaceutical industry. 2 brown h et al. Container closure integrity testing ccit is an assay that evaluates the adequacy of container closure systems to maintain a sterile barrier against potential contaminants. Container closure integrity testing ccit commonly referred to as leak detection is a non destructive packaging inspection system to maintain an aseptic barrier against potential contaminants.
Comparison to dye ingress tests. Container closure integrity of parenteral products is essential to protect the product through the entire lifecycle until patient delivery. Vacuum decay container closure integrity testing technology. The fda recommends performing a container closure integrity test in lieu of a sterility test as a component of a.
Eurofins biopharma product testing is committed to offering the most up to date methods for testing container closure systems for final drug product packaging we have invested in state of the art instrumentation to meet these regulatory guidelines and verify the safety of your container closure system. Contaminants that could potentially cross a container closure barrier include microorganisms reactive gases and other substances usp 1207. 3 wolf h et. Is a deterministic testing procedure so it is less subject to error is repeatable and gives quantitative and predictable results.
Container closure integrity testing. Pti s non destructive inspection technologies verify container closure system integrity with deterministic quantitative test methods for vials ampoules syringes. Container closure integrity testing is an assay that evaluates the ability of the container closure system to maintain a sterile barrier and prevent any leakage resulting in contamination of products.